‘Aducanumab’- it’s a long, hard-to-pronounce word that you not have heard of before. But for people affected by Alzheimer’s disease, it represents a hopeful new breakthrough. Developed by the company Biogen, Aducanumab is a potentially groundbreaking new drug. It aims at reducing cognitive decline to help people with Alzheimer’s.
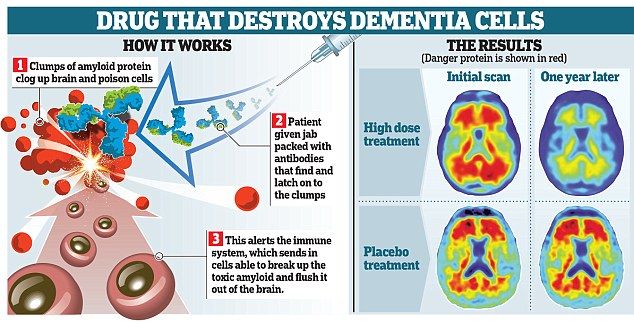
Unlike other drugs, Aducanumab was designed to actually slow or reverse the progression of Alzheimer’s. Based on the hypothesis that a buildup of beta-amyloid (a “sticky compound”) in the brain contributes to the development of Alzheimer’s. Aducanumab seeks to target beta-amyloid in order to curb neurodegenerative effects.
However, there are people who are hoping on Aducanumad. But there is also a fair share of skepticism about this drug. This is because of a startling reversal in Biogen’s own position when it comes to the effectiveness of the drug. In March 2019, Biogen declared that clinical trials of Aducanumab has been proven to be ineffective. And 7 months later, Biogen changed its tune. They claimed that “a larger data set showed that Aducanumab reduced clinical decline in patients with early Alzheimer’s disease.” Armed with this newly optimistic analysis, Biogen is now seeking FDA approval.
We are being cautioned against viewing Biogen’s data through rose-tinted glasses. Further trials are still needed. However, others, including many advocacy groups, are pushing for approval. They are eagerly awaiting the potentially beneficial drug to be accessible. Though there is no crystal ball for predicting the FDA’s verdict. Aducanumab will have a profound impact on both research and treatment of Alzheimer’s.



One Response
I pray to God’s will for the FDA’s to approve Aducanumab to stop this ugly Alzheimer’s effecting people life’s so that people like my mother can have there independence back to revoke the EPOA who has gone against there wishes by locking them up in a rest home for good.